Research
Organic Conducting Materials: Oligomers and Polymers
Acenes containing heteroatoms (heteroacenes) are a potentially valuable class of molecules for application in organic electronics. However, they are challenging to synthesize and current routes are inefficient. Thus, our strategy involves a two-step approach for the synthesis of novel N-heteroacene oligomers and polymers based on a precursor route. N-heteroacene precursors are synthesized efficiently and their cyclization to generate fused aromatic oligomers or polymers is virtually quantitative in yield. Current efforts include the synthesis of oligomers, with more than five rings, and polymers as well as their characterization.

Surface Modification: Polymeric Thin Films
Surface modifications of materials can be used for a variety of different applications including control of adhesion, protective coatings, and resistance of friction and wear. Our interest in surface modification utilizes polymers as a medium for exploring protein resistance. The polymeric surfaces we are interested in are polymer brushes and crosslinked thin films. Polymer brushes are monolayers of polymer chains attached to a surface at one end. Thin films can be deposited on a surface and then crosslinked using UV light. Most studies of polymer surfaces look at the native, as-fabricated form. However, it is also interesting to think about applications where removal or degradation of the polymer from the substrate is useful.
Dendrimers for Medical Applications
Drug Delivery to the Brain
Patients undergoing chemotherapy treatment experience a significantly diminished quality of life due to inefficient and non-specific drug delivery. The efficient delivery of chemotherapy agents to the central nervous system (CNS) has eluded scientists. The administration of drugs to the brain is significantly hindered by the blood brain barrier (BBB), which is a tightly packed lipophilic sheath encapsulating vasculature of the CNS. Dendrimers have shown promise as a molecular vehicle to shuttle chemotherapy drugs across the BBB, but traditional methods of encapsulation or conjugation do not offer significant improvements over unbound drug delivery.
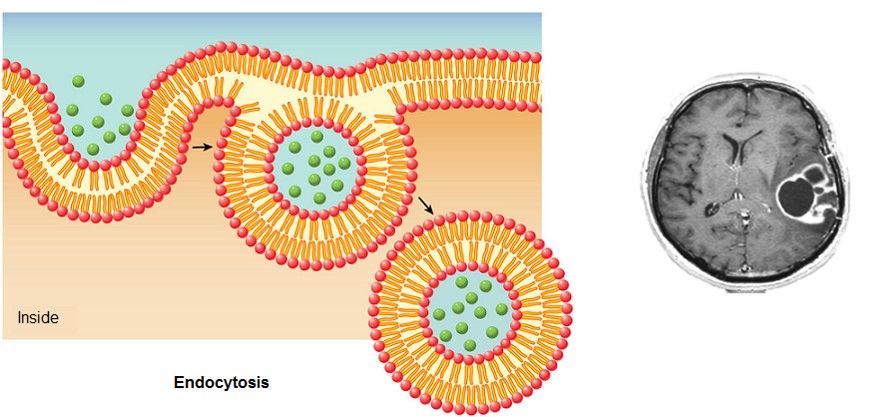
Our novel dendritic structure utilizes multiple pH sensitive covalent binding sites to embed the drug in the interior of the structure in order to shield the drug from non-specific interactions. In addition, the tunable architecture of the dendrimer allows for multiple avenues to cross the BBB including receptor mediated endocytosis or simple diffusion due to the molecular size of the dendrimer/drug complex.
Delivery of Genetic Material
Because of its highly specific nature, gene therapy has been gaining increased support as a means to treat disease. Utilizing a combination of peptide chemistry and microwave chemistry, efforts are being made to synthesize novel dendritic scaffolds and novel dendrimer cores towards applications in gene delivery; this DendriPep will incorporate tryptophan, beta-alanine, and a lysine derivative that will induce a permanent positive charge on the DendriPep.

Polymers for Phosphorus Capture
Phosphorous is not a sustainable element on our planet. Estimates suggest that mined phosphate will be depleted in 40-50 years. Meanwhile, phosphorous is an essential element for life and is increasingly becoming tied up either in minerals where it is not biologically available for uptake by plants or in waterways where it accumulates in lakes and ponds and promotes algal growth and eutrophication. This project will establish a systematic set of structure-property relationships for polymer-based phosphorous capture and recovery to determine the most proficient candidates. These candidates will then be studied in real world scenarios to find ways to make phosphorous a sustainable element.

